Background:
Autologous T cells engineered to express chimeric antigen receptors (CARs) targeting CD19 have shown rapid and durable responses in B cell malignancies. Although CD19 CAR-T cells have demonstrated remarkable success, CD19-negative relapses occur in 30-45% of patients, highlighting the need for adoptive immunotherapies with alternative targeting approaches. B-cell activating factor (BAFF) is a critical B cell survival factor. Receptors of BAFF (BAFF-R, TACI and BCMA) are expressed by a wide range of B cell neoplasms, including ALL, CLL, NHL and MM, making them attractive therapeutic targets. We developed a novel ligand-based CAR that when expressed in T cells, targets and eliminates malignant B cells expressing BAFF receptors (BAFF CAR-T). This approach has several potential advantages over CD19 targeting CAR-T therapy: CD19 is expressed on all B cells, but BAFF receptors are expressed only on mature B cells, making it a more specific antigen for targeting and potentially narrowing down the side effect profile. BAFF CAR-T cells are a potential therapeutic strategy to treat CD19 CAR-T relapsed patients as well as chemotherapy resistant patients.
Methods:
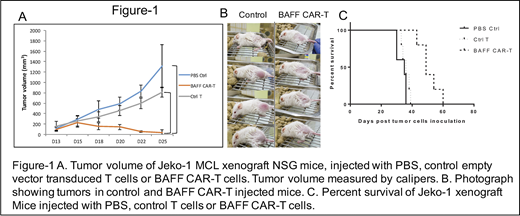
BAFF ligand was fused to a second generation CAR backbone containing 4-1BB costimulatory and CD3ζ intracellular signaling domains. T cells were isolated from human blood, activated and transduced with BAFF-CAR lentiviral particles. In vitro tumor cell killing was analyzed using calcein-AM cytotoxicity assay. For in vivo testing of BAFF CAR-T cytotoxicity, we used mantle cell lymphoma (MCL) Jeko-1 xenograft model. Immunocompromised NSG mice were subcutaneously injected with human MCL cell line Jeko-1 (10.106 cells at day 0). Once these mice developed measurable tumors, we injected T cells transduced with empty vector (control T cells) or BAFF-CAR T cells (10 x 106 cells) or PBS intra-tumorally as a one-time injection. Tumor volumes were measured every other day using calipers.
Results:
BAFF CAR-T cells showed significant cytotoxicity in vitro (not shown) and in vivo against human MCL cell line Jeko-1. Mice treated with BAFF-CAR-T showed significant reduction in tumor volume compared to mice treated with control T cells and PBS (Figure 1A, B). Tumor progression was observed after control T cell and PBS treatment, whereas the cohort treated with BAFF CAR-T did not show any tumor progression, and with complete or near-complete tumor eradication. Survival analysis showed the BAFF CAR-T treated cohort had significantly longer survival compared to control-T cell and PBS treated cohorts (Figure 1C). Mice were sacrificed when tumor volume reached 2 cm3.
Conclusion:
Our data suggest that targeting BAFF receptors with a novel, ligand-based BAFF-CAR-T is a feasible and effective immunotherapeutic strategy to eliminate malignant B cells, warranting further development. BAFF-CAR-T cells have therapeutic potential against a wide spectrum of B cell malignancies, including CD19 negative relapsed disease. Clinical grade expansion and clinical trials are in development for BAFF CAR-T therapy non Hodgkin lymphoma patients.
Parameswaran:Luminary Therapeutics: Consultancy; Luminary therapeutics: Research Funding. de Lima:Kadmon: Other: Personal Fees, Advisory board; BMS: Other: Personal Fees, advisory board; Incyte: Other: Personal Fees, advisory board; Celgene: Research Funding; Pfizer: Other: Personal fees, advisory board, Research Funding. Caimi:Amgen: Other: Advisory Board; Bayer: Other: Advisory Board; Verastem: Other: Advisory Board; Kite pharmaceuticals: Other: Advisory Board; ADC therapeutics: Other: Advisory Board, Research Funding; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal